People are living longer. New cures are being found for life-threatening conditions. Patients are ordering prescribed medications online and self-medicating at home. More medicines need constant temperatures in transit. Drug costs are rising. How is airfreight providing the ever-more-challenging link between producer and user?
The forwarder’s perspective
DHL Global Forwarding (DHL) is a world leader in pharma logistics; it now has over 100 pharma stations globally, where it maintains teams specially-trained in handling pharma traffic. 30+ key life science facilities in this network have been certified according to the IATA CEIV Pharma standard. Stations have the necessary infrastructure and facilities, such as cool rooms and temperature-controlled storage, while DHL cooperates for its first- and last-mile transportation with carefully-controlled service providers managed and monitored under its GDP-certified procedures.
“There has been a huge increase in awareness of pharma logistics requirements over the past ten years,” says Nina Heinz, the company’s Global Head of Network & Quality, Temperature Management Solutions, who works with her team to cover a wide array of quality matters in regulatory compliance, audits, training, partner management, customer complaints and continuous improvement.
“Investment has grown massively, with a lot of this focused on Europe. We have also undertaken wide-spread certification over the past three to four years; it is this certification process which is driving improvement in the industry as a whole.”
IATA CEIV Pharma: a common language
Initially, DHL Global Forwarding focused on external GDP certification, but is now shifting its emphasis and support to the IATA CEIV Pharma certification, which it sees as more appropriate to air cargo. “IATA CEIV Pharma has been tailor-made for air transportation and air cargo”, says Nina, “Therefore it takes into account elements such as tarmac transit times and is effectively a common language for us all.”
Shippers now insist on certification of their logistics providers to GDP as a minimum, but they also commonly conduct their own complementary audits. The wider adoption of IATA CEIV Pharma, with its more comprehensive scope, could help to reduce the number of these audits required, DHL believes.
“We really need greater transparency from the other elements of the supply chain,” continues Nina. “For example, it would be helpful to know what pharma handling facilities are available on an airport-by-airport basis. But we also need more real-time information on shipment status: is it in the cool room? What’s the temperature? The air cargo industry is fragmented, and is lagging behind in technological terms.”
While the technology exists to track shipments and log and transmit condition data, progress is being held back by the confusing number of solutions being offered, and the fact that any active devices need approval for use on aircraft. “There are too many devices in circulation; IATA is playing a lead role, but we need discussion and agreement from all interested parties on universally-acceptable standards and solutions.”

Risks on the ramp
Improvement is also necessary on the ramp, where much of the scope for temperature excursions exists. “We need to reduce the risks caused by the local ambient conditions, which means streamlining processes and reducing ramp times.”
Does Nina feel that air cargo could lose out to ocean freight in the pharma shipping stakes? “No, I don’t see a threat of modal shift,” she continues. “There will always be air traffic, and there will always be ocean traffic; they are different worlds.”
Security is one of the biggest headaches for the pharma industry. The high value of all pharmaceuticals makes them a tempting and lucrative target for theft, but also for forgery. “There is an increasing focus on serialization to counteract product fraud,” says Nina, “but we observe still a slight discrepancy between what the industry needs and what is currently offered. We at DHL Global Forwarding are therefore working very closely and in an open dialogue with our customers to explore and meet their needs.”
“Whereas manufacturers were based in markets like the USA, and supplied their own market, goods are now moving in from Asia because of cost. That has created a greater awareness of the need to maintain temperatures from manufacturer to patient.”
A shipper speaks
Ryan Viegas is the Head of Logistics, APAC, for Teva Pharmaceutical Industries, based in Mumbai. Teva specializes primarily in generic drugs, but also supplies active pharmaceutical ingredients and proprietary pharmaceuticals. It is the largest generic drug manufacturer in the world and one of the 15 largest pharmaceutical companies, with facilities in Israel, North America, Europe, and South America.
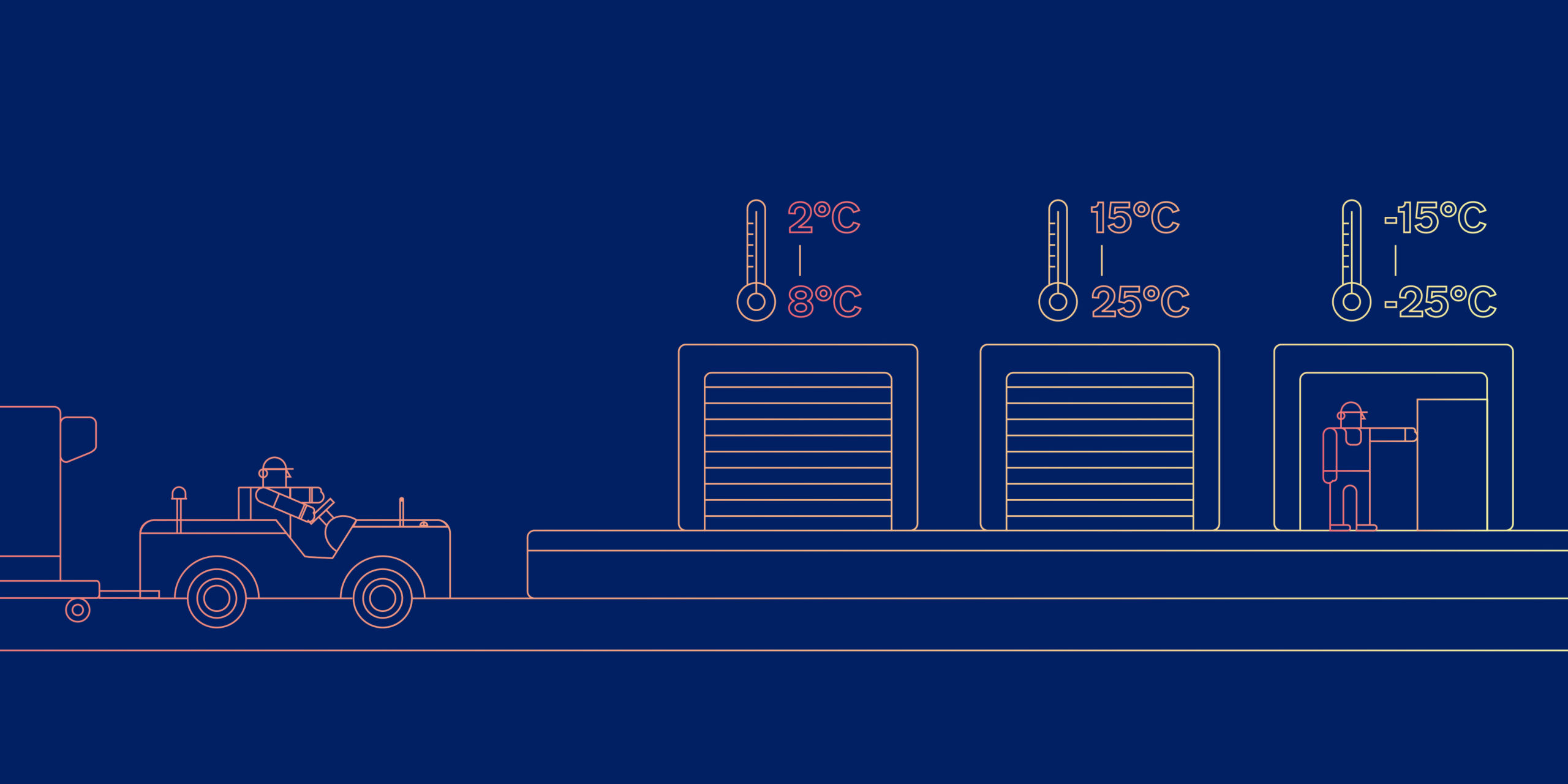
100% of Ryan’s traffic requires temperature control: either 15°C-25°C, or 2°C-8°C. Goods that are allowed to fall outside their temperature bands can lose some or all of their efficacy, and could (in extreme cases) present a hazard to patients.
For the 15°C-25°C shipments, Teva generally uses passive temperature control measures such as thermal blankets; it employs active thermal containers only for the 2°C-8°C traffic.
“The supply chain has been undergoing major change over the past ten years,” says Ryan. “Whereas manufacturers were based in markets like the USA, and supplied their own market, goods are now moving in from Asia because of cost. That has created a greater awareness of the need to maintain temperatures from manufacturer to patient.”
To add complexity, some active ingredients originate in Europe, and are transported to Asia for the manufacturing process, before finished product is exported to markets as far apart as the USA, Europe and South America. “That means certification of processes from manufacturer to patient through GDP is very important.”
“Straight to patient is the future, and there’s a lot of discussion about it.”
Straight-to-patient is the future
Teva ships to a wide variety of consignees, including distributors, hospitals and clinics. But Ryan sees the much-publicised straight-to-patient concept as the way forward: “Straight to patient is the future, and there’s a lot of discussion about it. If a patient is taking a particular medication on a regular basis, he should be able to go online and re-order, and the system could also issue reminders to re-order. That would be a great way forward.”
Given that such a concept would have a lot in common with the kind of online shopping systems that are now commonplace in most markets and commodities, it’s little surprise that the pharma manufacturing sector is in discussion with leading e-commerce organisations about the possibility of forming a specialised pharma e-commerce joint venture.
What’s Ryan’s view about how well the air cargo industry is matching up to expectations and requirements of companies like his? “Some players are implementing very good temperature controls,” he says. “But others still view a pharma shipment as just boxes. Many of the current complaints from my industry are valid.
“Air transportation is about speed, as well as compliance. Transhipment is a problem; at some airports, this causes gaps with zero visibility; even the forwarder cannot see what is happening. What we really want is real-time monitoring, and we are looking at ways around the problem with IATA.”
The airline angle
Air France KLM Martinair Cargo (AFKLMP) has spent some twenty years transporting pharmaceuticals, but its focus has been even greater in the past four years or so. The airline obtained IATA CEIV Pharma accreditations for Air France, KLM, and separately also for its two hubs at Paris Charles de Gaulle and Schiphol in 2016. Now, it participates in all major conferences and trade associations to obtain as much feedback as possible from pharma shippers, to see where it needs to invest for the future.
AFKLMP offers many different specialist products, including “Closed and Controlled Cool Chain Solutions” for time & temperature pharma cargo. “We safeguard temperature requirements throughout the supply chain working with packaging partners such as Csafe Global, Va-Q-Tec, Envirotainer, Skycell etc. so we can provide active or passive solutions,” says Beatrice Delpuech, VP Verticals, AFKLMP Cargo.
“We try to find the best option for the customer, and then we ensure the supply chain maintains the customer’s chosen temperature which are generally 2°C-8°C, 15°C-25°C and 2°C-25 °C. We have to be very accurate with the processes, and that means not only during the flight - so we have to pass the correct information to the handler and the aircraft crew. The whole network must be suitable for the product according to the specification; we transport these very sensitive shipments only when we know we can do it.”
Warsaw Convention offers limited protection
Avoiding mistakes is of paramount importance for the shipper customer, as he is currently only protected to the limits set by the Warsaw Convention rules - which represent only a fraction of the real value of any pharma shipment.
Beatrice reports that Control 15-25 is showing the best growth at present, and expectations for service levels are constantly increasing. As yet, though, straight-to-patient is not in demand. But Beatrice and her team are looking at how e-commerce may also become a way of purchasing medications.
“The ability to handle across the network is something very important to us, in order that we can provide the right service to the customer.” That said, “In cases where a station is not prepared, we can always create a custom solution,” she adds. But she says that the handler’s capabilities are very important, and that this is one of the criteria contained in handling agent contract tenders.
Beatrice says GDP is a good standard, “But for us it was not sufficient. So we decided to be certified for IATA CEIV Pharma at both hubs, and for both Air France and KLM. It is a standard recognized by our customer, the shipper, and we couldn’t be at the necessary level of understanding and sharing of information without it.” AFKLMP was one of the first to be certified, in 2016, and it maintains constant checks between audits, as well as undergoing regular audits from customers.
On the eternal question of visibility, Beatrice says that information is available in many different places, “But to be more competitive we need to gather this information and make it available to the outside world. The GHA also needs to be proactive as they are our representative at many locations.

“Hactl was one of the first in the world (and the first in Hong Kong) to acquire WHO GDP certification as soon as the standard was devised. Later, it was the first to achieve IATA CEIV Pharma certification in Hong Kong.”
Digital solution for remote control
“We are working on a live digital solution for the customer, in order to speed up responses, and control the temperature of containers. In many places in the customer journey, we are already recording the temperature information, and will be able to provide this as well as the location.”
Shippers and their agents could help to make pharma supply chains better by providing more forecast information. “Capacity is often constrained. What could help is when they book a shipment, to have an accurate forecast of the shipment, including dimensions. Packaging is also very important. We are working closely as much as we can with associations to communicate this to shippers.”
She concludes: “Pharma is one of the biggest focuses we have, and we will maintain this for the coming years. Now everybody is coming into the business, but for us it has always been a top priority and will remain so.”
Handling plays a vital role
As the handling agent for over 100 airlines at the world’s number one air cargo hub, Hong Kong, Hactl has long been aware of its vital role in pharma supply chains. In the early days of pharma by air, it invested heavily in modifying cool room facilities and staff training to ensure that it could provide robust support for airline customers wishing to develop this lucrative new revenue stream.
Typically, though, Hactl didn’t stop there: it was one of the first in the world (and the first in Hong Kong) to acquire WHO GDP certification as soon as the standard was devised. Later, it was the first to achieve IATA CEIV Pharma certification in Hong Kong. In every audit since, it has achieved 100% fault-free scores. Its pharma certifications and procedures are underpinned by substantial further investments in facilities and equipments, such as thermal dollies that eridcate temperature excursions on the ramp, even in extreme temperature conditions, and in the establishment of its legandary “Golden Route” fast-track for pharma cargo handled through its facility.

